Evaluating A Stroke Prevention Device
Ischemic Stroke is the most frequent type of stroke and is subject to many studies investigating prevention means. Avoiding the difficulties and ethical problems of experimental in-vivo research, in-vitro testing is a convenient way of studying in controlled conditions the morphological impact and mechanical aspects of emboli dynamics. This in-vitro study was performed with two realistic silicone aortic-arch phantoms submitted to physiological pulsatile flow conditions. In the in-vitro test bed, using automatic image tracking and analysis, it was made possible detecting and tracking artificial spherical emboli candidates circulating in the anatomic aortic-arch models under a realistic based-patient blood flow profile. The emboli trajectories as well as their repartition in the different supra-aortic branches are presented for the two aortic-arch geometries obtained from CT scans. Through a statistical analysis performed with several artificial emboli sizes, the experimental study shows that the repartition percentages of the emboli closely follow the flowrate repartition percentages for both aortic-arch models, suggesting that higher flowrates lead to higher concentrations of emboli in a given artery. Sets of human thrombi were also injected and the repartition percentages have been established, giving the same trend as for artificial emboli.
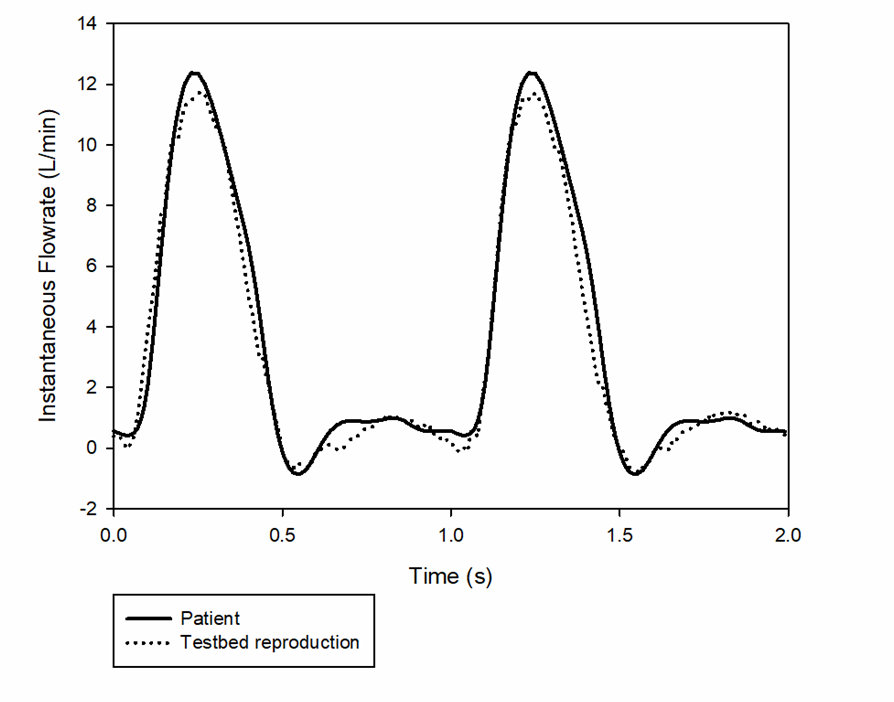
Physiological Flow Reproduction
Pulso pulse duplicator was programmed to replicate physiological aortic flow signals in the aortic-arch anatomical silicone model. The device allowed to reproduce normal, accelerated and pathological flow conditions. Data collected from the study were used to validate numerical simulations of a stroke prevention device.
